About AMP® PD Overview
About the Accelerating Medicines Partnership® (AMP®) Program
![]()
The Accelerating Medicines Partnership® (AMP®) program is a public-private partnership between the National Institutes of Health (NIH), multiple biopharmaceutical and life sciences companies, and non-profit organizations.
Managed through the Foundation for the NIH (FNIH), AMP aims to identify and validate the most promising biological targets for therapeutics. Disease areas covered by AMP at the launch of this site include Alzheimer’s disease, type 2 diabetes, the autoimmune disorders of rheumatoid arthritis and systemic lupus erythematosus (lupus) and Parkinson’s disease. Additional disease areas are being evaluated for addition to AMP.
The Accelerating Medicine Partnership in Parkinson’s Disease (AMP PD)
In 2018, through FNIH, an AMP partnership between the National Institute of Neurological Disorders and Stroke (NINDS), National Institute on Aging (NIA), the Food and Drug Administration (FDA), GSK, Pfizer, Sanofi, Bristol-Myers Squibb, Verily and the Michael J. Fox Foundation for Parkinson's Research (MJFF) was launched for Parkinson’s disease (PD). Aligning Science Across Parkinson's (ASAP) initiative joined the partnership in 2019 and AbbVie joined the partnership in 2022 to expand project goals.
The research plan proposed for the AMP program in PD encompasses a deep molecular characterization and longitudinal clinical profiling of PD patient data and biosamples with the goal of identifying and validating diagnostic, prognostic and/or disease progression biomarkers for PD.
A critical component of this partnership is broad sharing of the AMP PD data and analyses with the biomedical community to advance research in PD. AMP PD utilizes well characterized cohorts with existing biosamples and clinical data that were collected under comparable protocols and using common data elements.
The Release 1.0 cohorts include the MJFF and NINDS BioFIND study, Harvard Biomarkers Study (HBS), the NINDS Parkinson's Disease Biomarkers Program (PDBP), and MJFF Parkinson’s Progression Marker Initiative (PPMI). Release 2.0 adds the following cohorts; NIA International Lewy Body Dementia Genetics Consortium Genome Sequencing in Lewy body dementia case-control cohort (LBD), the MJFF LRRK2 Cohort Consortium (LCC), and the NINDS Study of Isradipine as a Disease Modifying Agent in Subjects With Early Parkinson Disease, Phase 3 (STEADY-PD3). Release 2.5 adds the MJFF and NINDS Study of Urate Elevation in Parkinson’s Disease, Phase 3 (SURE-PD3). Release 3.0 adds the Global Parkinson’s Genetics Program (GP2). Release 3.0 also adds additional targeted proteomics from PPMI and PDBP as well as new bulk exRNA pilot sample data, new WGS single sample data, and a new global inventory table. Release 4.0 adds Postmortem Brain Sequencing, Untargeted Proteomics Data, and normalized Targeted Proteomics Data.
Thank you to all of the research participants who have contributed data through BioFIND, GP2, HBS, LBD, LCC, PDBP, PPMI, STEADY-PD3, and SURE-PD3 and to all of the study teams who have collected and managed these data. AMP PD appreciates your contributions, which are fundamental to AMP PD resource development.
AMP PD Consortium
Government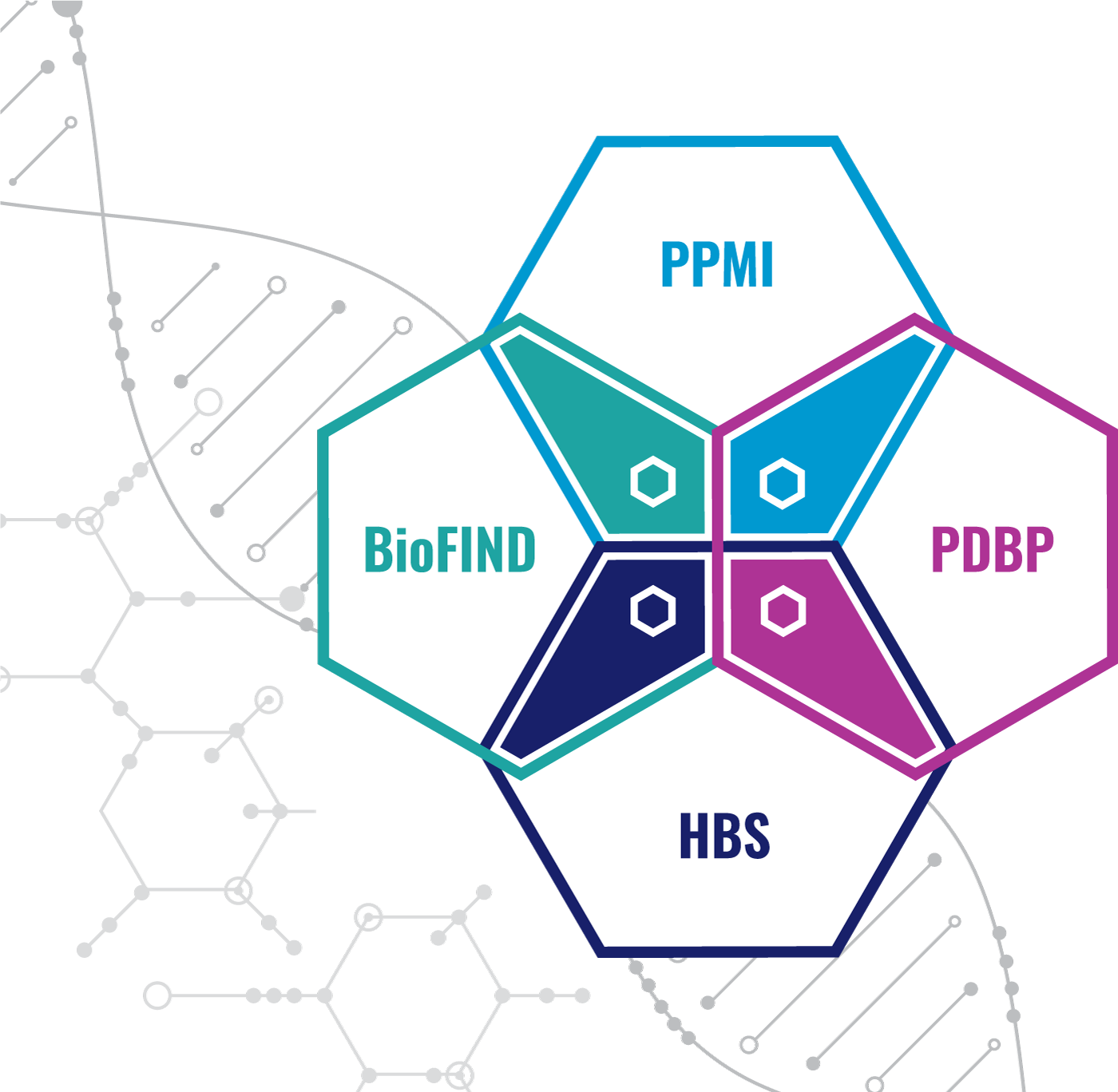
Food and Drug Administration (FDA)
National Institute of Neurological Disorders and Stroke (NINDS)
National Institute on Aging (NIA)
Industry
Nonprofit
Aligning Science Across Parkinson's (ASAP) Initiative
The Michael J. Fox Foundation (MJFF)
Data
MJFF LRRK2 Cohort Consortium (LCC)
Brigham and Women's Hospital/MGH Harvard Biomarkers Study
NINDS Parkinson's Disease Biomarkers Program
MJFF Parkinson’s Progression Markers Initiative
The MJFF and NINDS Study of Urate Elevation in Parkinson’s Disease, Phase 3 (SURE-PD3)
Global Parkinson's Genetics Program (GP2)
Managing
The FNIH, managing partner for AMP PD, is actively recruiting new industry and nonprofit partners. Inquiries about how your organization can join the partnership should be submitted to Tyler Fortuna, AMP PD Project Manager, at tfortuna@fnih.org.